Depending on their intended use, medical devices may be subject to different cleanliness requirements. In addition, there are several important aspects that must be taken into account to ensure the safety of the product throughout its entire service life.
Bioburden
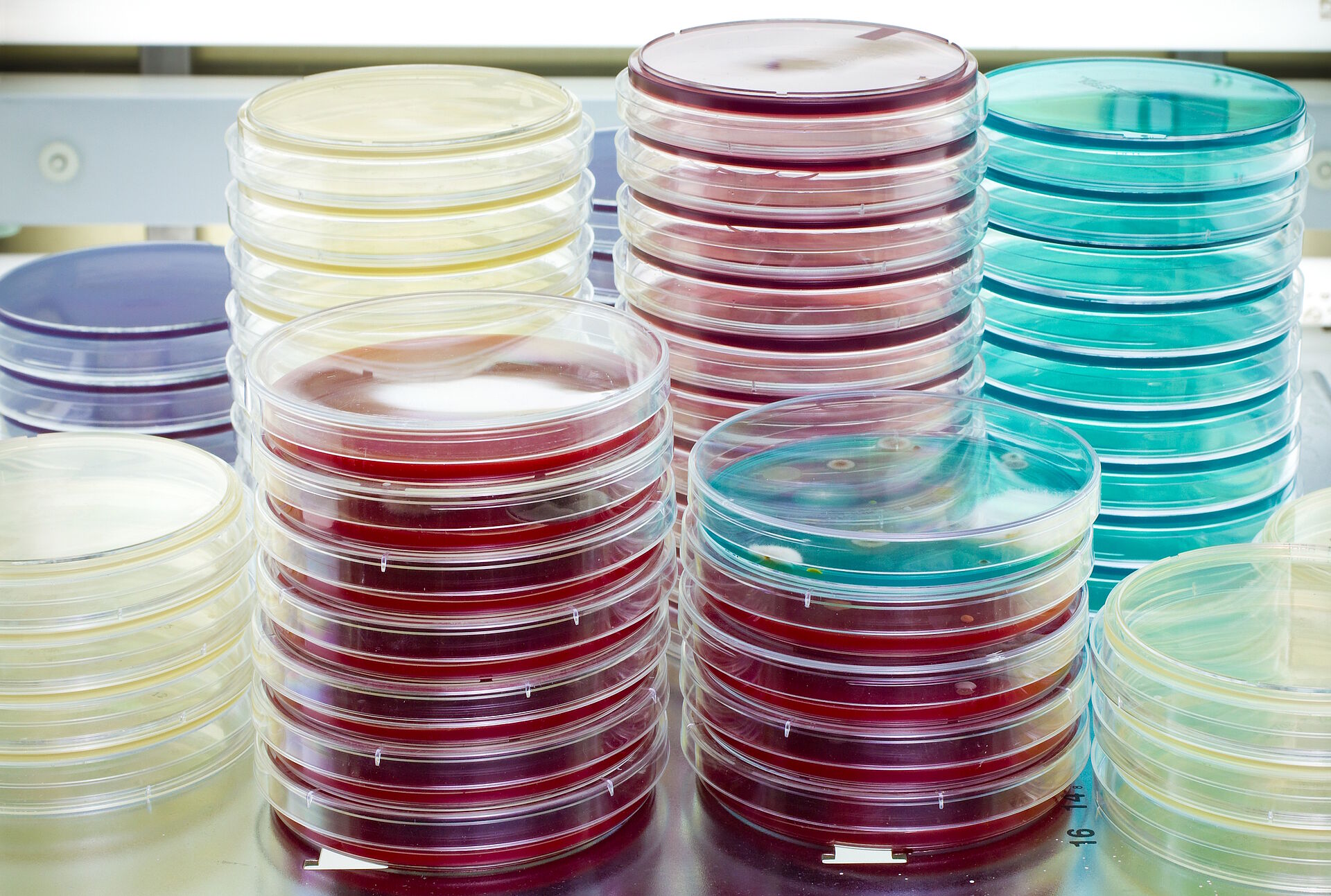
The germ load, also known as bioburden, is the total number of microorganisms on a medical device.
It is determined in accordance with DIN EN ISO 11737-1 by means of microbiological tests in which germs are rinsed from the surface of the product and incubated on culture media.
Further details for download
Processing of medical devices
Detection of bacterial endotoxins
The LAL test (Limulus amoebocyte lysate test) according to Ph. Eur. 2.6.14, ANSI/AAMI ST72 and DIN EN ISO 11737-3 quantifies fever-inducing endotoxins, which are components of the cell wall of Gram-negative bacteria.
This test is important for the evaluation of medical devices that come into contact with the bloodstream or the cardiovascular system.

Evidence of contamination (TOC, THC)
THC (total hydrocarbon) and TOC (total organic carbon) determination are essential analyses for checking the cleanliness of medical devices.
They measure organic residues from process aids such as oils, fats, lubricants or cleaning agents.
Particle measurements or the analysis of surface properties using microscopic methods can also provide information about contaminants.

Microbial Ranking of Packaging Materials
Requirements for packaging for sterile medical devices are described in DIN EN ISO 11607-1. ASTM F1608 tests the ability of the material to prevent the penetration of bacteria and thus demonstrates the microbial barrier properties of porous packaging materials for sterile medical devices.

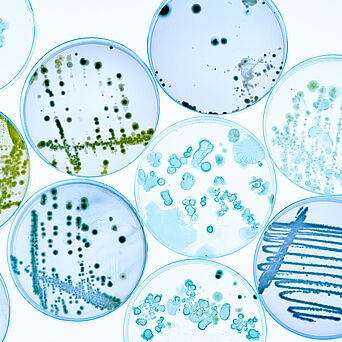
Hygiene Monitoring in the Production Environment
Hygiene monitoring involves checking the microbiological quality of air, surfaces and water in the production environment. These measures are crucial for compliance with hygiene standards and product safety.